Abstract
Background:
The relevance of autologous hematopoietic cell transplantation (ASCT), a crucial part of multiple myeloma (MM) therapy for eligible patients, has been questioned in light of improvements in induction therapy that result in excellent responses. To increase progression free and overall survival, it is crucial to continue developing first-line treatments using non-ASCT and ASCT-based methods. In addition, it is important to determine if a specific patient population might benefit from a given strategy to develop personalized cancer care based on risk stratification. In our meta-analysis, we assessed the efficacy outcomes of HDT/ASCT in combination with standard induction treatment as compared to standard-dose therapy alone for newly diagnosed MM.
Materials & Methods:
A comprehensive literature search was carried out on PubMed (Medline), EMBASE, CENTRAL (Cochrane Library), and Google Scholar from inception till 20th July 2022. HDT/ASCT versus standard-dose therapy (SDT) employing novel therapies was evaluated in phase 3 RCTs. The inclusion criteria were randomized controlled trials (RCTs) that directly compared combination chemotherapy followed by consolidation with HDT/ASCT(high-dose therapy with melphalan followed by autologous stem cell transplant) to SDT(Standard Dose therapy) alone without ASCT in patients with newly diagnosed MM(Multiple Myeloma). We included papers which reported PFS and OS as their endpoints using intention-to-treat analysis and had a minimum of 2 years of follow-up. All the original studies, review articles, and clinical trials with a sample size of <100 were excluded from the review. Review Manager Software (RevMan, version 5.3) was employed for statistical analyses, and forest plots were created to analyze the results visually. The Mantel-Haenszel random effect model was applied. Higgins's I2 test was used to evaluate heterogeneity across the studies.
Results:
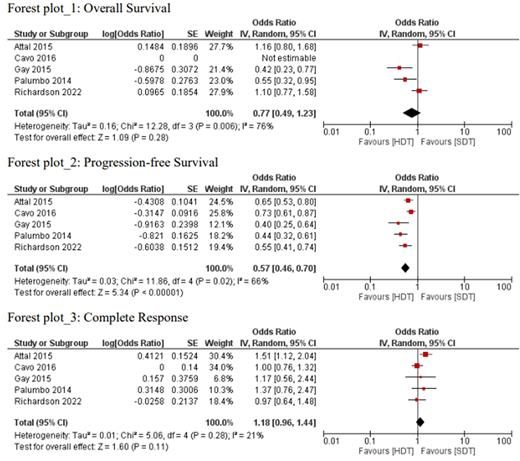
A total of five Randomized Controlled Trials consisting of 3,294 participants were included in the meta-analysis. Only four studies included in the analysis reported OS. The pooled analysis reported no significant benefit for overall survival in the HDT/ASCT group compared to SDT (OR: 0.77, 95% CI; 0.49, 1.23, I2= 76%, P=0.28); however, the results were limited by significant heterogeneity. (Forest Plot 1). The combined odds ratio(OR) for complete response(CR) was 1.18 (OR: 1.18, 95%CI; 0.96-1.44, I2=21%, P= 0.11) with SDT compared with HDT/ASCT, although it is not statistically significant. (Forest Plot 3). Similarly, the combined odds ratio for a complete response did not favor any treatment group (OR: 1.18, 95%CI; 0.96-1.44, I2=21%, P= 0.11). All five studies reported PFS. Our results indicated statistically significant PFS in HDT/ASCT group as compared to the SDT alone group (OR: 0.57, 95% CI; 0.46-0.70, I2= 66%, P <0.00001). (Forest Plot 2)
Conclusion:
HDT/ASCT was linked with a better PFS as compared to SDT without ASCT in newly diagnosed multiple myeloma. The OS was comparable for both HDT/ASCT and SDT without ASCT in all RCTs. Overall survival for multiple myeloma patients has gradually improved over the last two decade. Overall survival benefit will be difficult to demonstrate between the two approaches as new and better treatments are getting available for salvage and bridging multiple myeloma patients to receive ASCT. ASCT in any line of treatment results in deeper remission and better outcomes, as well as equalizes the PFS benefit seen in using ASCT in first line treatment. Based on data from our meta-analysis, use of HDT/ASCT should be individualized based on disease risk group and patient's preference.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal